These silicon oil droplets have an odd quality to them, and it has nothing to do with the way they bounce over the petri dish. They truly mimic many of the bizarre quantum mechanical processes, although on a scale that we can actually observe. And this Veritasium episode explores how that can aid in our understanding of some of the wilder theories relating to quantum particles.
But first, let's ask ourselves why these oil droplets are
even bouncing in the first place. Derek from Veritasium has positioned a petri
dish filled with silicon oil on top of a speaker in the experiment above, and
he is vibrating the dish with the speaker.
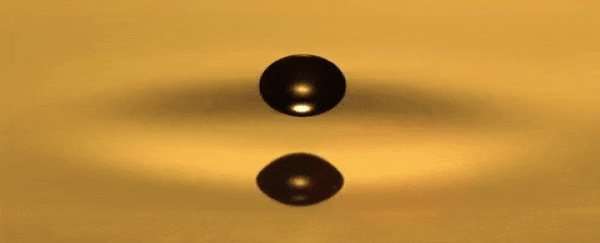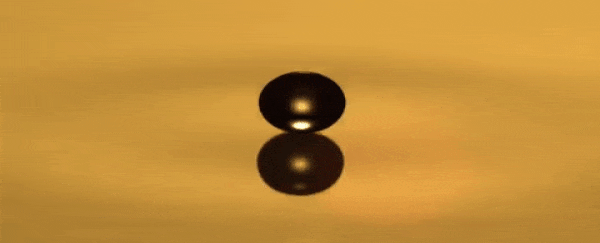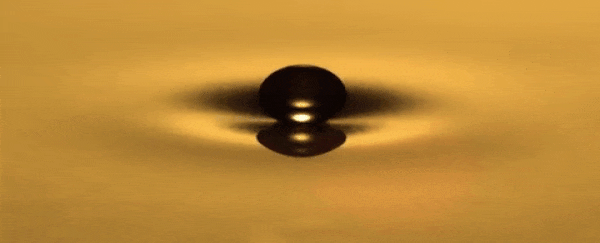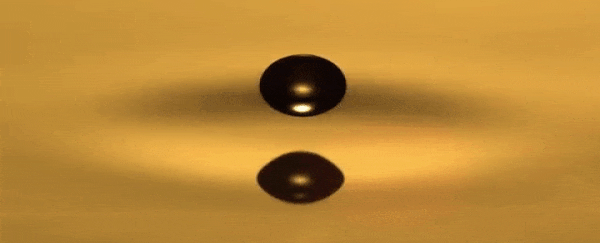
He can use a toothpick to create droplets that bounce over a
thin layer of air between the oil and the droplet that never becomes too small
for the oil to recombine, hovering around the surface.
A standing wave that oscillates up and down is produced in
the dish each time the droplet bounces.
The standing wave is not just produced by the droplet; it
also interacts with it on its subsequent bounce. Additionally, if it keeps
touching down on the same side of the wave, the droplet is propelled ahead and
is referred to as a "walker" by scientists.
But how these droplets act is what's really fascinating.
Although they are around 1 millimetre wide and far too large to be quantum,
researchers have just lately learned that they can utilise these tiny droplets
to mimic many of the peculiar effects of quantum physics.
Consider the traditional double-slit experiment as an
illustration. The double-slit experiment, which is used in classical quantum
mechanics, involves directing a stream of particles, such as electrons, towards
two small slits.
On the other side, as opposed to producing two discrete
clusters of electrons behind the slits as you might anticipate, they instead
form an interference pattern, or a very equal scattering of electrons.
That is only one of the many puzzling events in quantum
physics; it occurs even when you send the electrons through one at a time.
You may do the double-slit experiment with these bouncing oil
droplets and see how the standing wave (also known as the pilot wave) passes
through both slits but the droplet itself only passes through one.
But since they are jostled and steered by these standing
waves, the droplets don't always go in a straight path. As a result, the
droplets end up dispersed on the other side in a pattern that closely resembles
a quantum interference pattern.
That's strange enough, but the droplets also exhibit the same
quantum tunnelling phenomena, which allows a particle to pass through a wall
that it wouldn't have the energy to cross in a classical universe.
The chaotic movement of an oil droplet known as a
"walker" contained in a circular corral, such as a petri dish, can be
observed over time as it bounces off its standing waves. This allows you to
create a probability density that displays the probability that the droplet
will be found at any given location within the petri dish at any given time.
That pattern will, it turns out, resemble the probability
density of electrons contained in a quantum corral quite a bit. Which is
strange, but obviously not a coincidence.
Why then do these droplets resemble quantum particles so
much?We'll let Derek explain that in the video above, but let's just say that
observing these little bouncing droplets can help physicists (and the rest of
us) wrap our tiny human minds around some of the competing hypotheses out there
about quantum mechanics, such as the Copenhagen interpretation and pilot wave
theory.
Check it out and see which you think makes more sense - or
just marvel at strange bouncing oil droplets that seem to defy physics. You do
you.
And if you want to find out more about how oil droplets - and
even water - levitate in the first place, check out the incredible episode of
Smarter Every Day below… he even makes them happen in space:







No comments